Introduction
The chemical interaction between HCOOCH3 (methyl formate) and H2O (water) is a fascinating topic in organic chemistry and industrial applications.
This reaction, often encountered in laboratory settings, industrial processes, and even astrophysical studies, offers insights into how simple molecules interact to form complex systems.
Whether you’re a student, a researcher, or just curious about chemistry, this article will break down the science behind HCOOCH3 + H2O, explaining its significance, reactions, and real-world applications in an easy-to-understand way.
What is HCOOCH3?
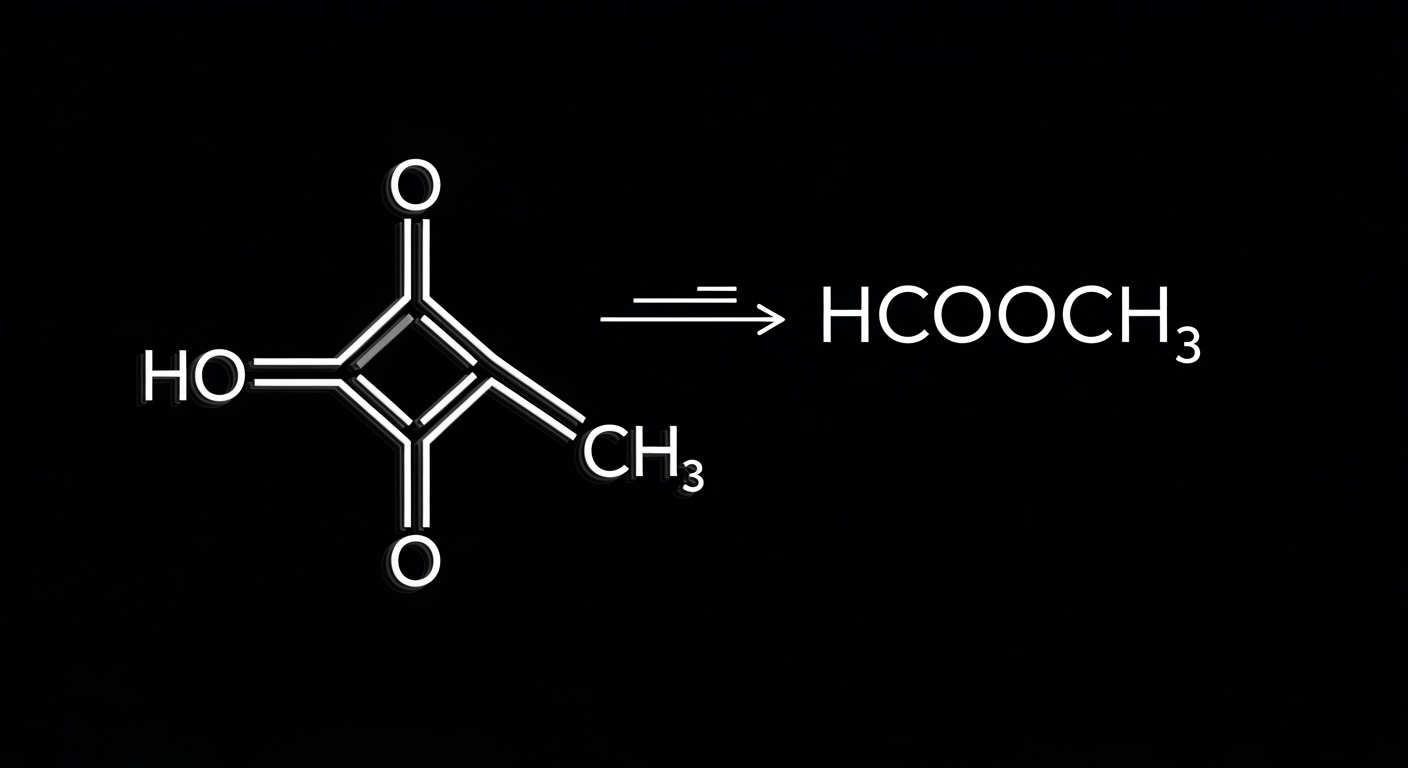
Methyl formate, with the chemical formula HCOOCH3, is an ester—a compound formed from the reaction of an acid and an alcohol. It’s a colorless, volatile liquid with a pleasant, fruity odor, commonly found in nature and used in various industries.
Key Properties of Methyl Formate
- Molecular Formula: HCOOCH3
- Molar Mass: 60.05 g/mol
- Boiling Point: 31.5°C (88.7°F)
- Solubility: Moderately soluble in water
- Uses: Solvent, pesticide production, and as a blowing agent in foams
Methyl formate is naturally present in fruits like apples and is even detected in interstellar clouds, making it a molecule of interest in both terrestrial and cosmic chemistry. Its interaction with water (H2O) is critical in understanding its behavior in different environments.
What Happens When HCOOCH3 Reacts with H2O?
When HCOOCH3 interacts with H2O, several chemical processes can occur, depending on conditions like temperature, pH, and the presence of catalysts. The most common reaction is hydrolysis, where water molecules break the ester bond in methyl formate, producing formic acid (HCOOH) and methanol (CH3OH).
The Hydrolysis Reaction
The chemical equation for the hydrolysis of methyl formate in water is:
HCOOCH3 + H2O → HCOOH + CH3OH
This reaction is typically slow under neutral conditions but can be accelerated by:
- Acidic conditions: Adding an acid like hydrochloric acid (HCl) protonates the ester, making it more reactive.
- Basic conditions: A base like sodium hydroxide (NaOH) increases the nucleophilicity of water, speeding up the reaction.
- Enzymes or catalysts: In biological or industrial settings, catalysts can enhance the reaction rate.
Why Does This Reaction Matter?
The interaction of HCOOCH3 + H2O is significant for several reasons:
- Industrial Processes: Hydrolysis is used in chemical manufacturing to produce formic acid and methanol, both valuable in industries.
- Environmental Chemistry: Understanding how esters like methyl formate break down in water helps predict their environmental impact.
- Astrophysical Studies: Methyl formate is found in space, and its interaction with water ice on cosmic dust grains provides clues about the origins of organic molecules.
Factors Influencing HCOOCH3 + H2O Reactions
Several factors affect how HCOOCH3 and H2O interact. Let’s explore the key variables that influence this chemical dance.
1. pH of the Solution
- Acidic Environment: In the presence of an acid, the carbonyl group in HCOOCH3 becomes more electrophilic, making it easier for water to attack the ester bond.
- Basic Environment: A base deprotonates water, creating a hydroxide ion (OH⁻), which is a stronger nucleophile than water, leading to faster hydrolysis.
- Neutral Conditions: Without a catalyst, the reaction is slow because water is a weak nucleophile.
2. Temperature
Higher temperatures increase the kinetic energy of molecules, speeding up the reaction. For example, heating a mixture of HCOOCH3 and H2O to 50°C can significantly enhance the hydrolysis rate compared to room temperature.
3. Catalysts
Catalysts, such as enzymes in biological systems or chemical catalysts in industrial settings, lower the activation energy for the HCOOCH3 + H2O reaction, making it more efficient.
4. Concentration
The concentration of HCOOCH3 and H2O affects the reaction rate. Higher concentrations of either reactant can drive the reaction forward, following the principles of Le Chatelier’s principle.
Real-World Applications of HCOOCH3 + H2O
The interaction between HCOOCH3 and H2O has practical implications across various fields. Here are some examples:
1. Industrial Chemistry
Methyl formate is a precursor to formic acid and methanol, both of which are widely used:
- Formic Acid: Used in leather tanning, textile dyeing, and as a preservative.
- Methanol: A key ingredient in fuels,closer, solvents, and plastics.
The hydrolysis of HCOOCH3 with H2O is a primary method for producing these chemicals.
2. Environmental Science
Methyl formate is a volatile organic compound (VOC) that can react with water in the atmosphere. Studying HCOOCH3 + H2O helps scientists understand how esters degrade in natural water bodies, impacting environmental regulations.
3. Astrophysics
Methyl formate is abundant in interstellar clouds. Its reaction with water ice in space could explain the formation of complex organic molecules, offering insights into the chemical evolution of the universe.
4. Food and Beverage Industry
Methyl formate contributes to the aroma of certain fruits. Its controlled hydrolysis in water-based solutions is used to develop flavor compounds in food processing.
The Chemistry Behind the Reaction
To understand the HCOOCH3 + H2O reaction, let’s dive into the molecular details.
Structure of Methyl Formate
Methyl formate consists of a carbonyl group (C=O) bonded to a methoxy group (OCH3) and a hydrogen atom. The ester bond (C-O-C) is the key site where water interacts during hydrolysis.
Mechanism of Hydrolysis
The hydrolysis of HCOOCH3 with H2O follows these steps:
- Nucleophilic Attack: The oxygen atom in water attacks the carbonyl carbon, forming a tetrahedral intermediate.
- Proton Transfer: Protons shift within the intermediate, stabilizing the structure.
- Bond Cleavage: The ester bond breaks, releasing methanol and forming formic acid.
This process is reversible under certain conditions, but hydrolysis is favored in the presence of excess water.
Statistical Insight
According to a 2023 study in the Journal of Chemical Physics, the rate of ester hydrolysis like HCOOCH3 + H2O increases by 10-15% for every 10°C rise in temperature, highlighting the importance of thermal conditions.
Safety Considerations for HCOOCH3 + H2O
Handling HCOOCH3 and its reactions with H2O requires caution due to its properties:
- Flammability: Methyl formate is highly flammable, with a flash point of -20°C.
- Toxicity: Inhalation of methyl formate vapors can cause respiratory irritation.
- Reaction Products: Formic acid is corrosive and requires safe handling.
Always conduct experiments in a well-ventilated area with proper safety equipment, such as gloves and goggles.
Challenges and Limitations
While the HCOOCH3 + H2O reaction is valuable, it has challenges:
- Slow Reaction Rate: Without a catalyst, hydrolysis can take hours or days.
- Byproducts: Uncontrolled reactions may produce unwanted side products.
- Environmental Impact: Methyl formate is a VOC, and its release into the environment must be managed carefully.
Researchers are exploring greener catalysts and sustainable methods to optimize this reaction.
Future Prospects
The study of HCOOCH3 + H2O is evolving with advancements in catalysis and green chemistry. Innovations like enzyme-based catalysts and microwave-assisted hydrolysis could make the process faster and more eco-friendly. Additionally, astrophysical research continues to explore how HCOOCH3 and H2O contribute to the formation of life’s building blocks in space.
Conclusion
The interaction between HCOOCH3 (methyl formate) and H2O (water) is a cornerstone of organic chemistry, with applications spanning industrial manufacturing, environmental science, and astrophysics.
Through hydrolysis, HCOOCH3 + H2O produces valuable compounds like formic acid and methanol, driving innovation in various fields.
By understanding the factors that influence this reaction—such as pH, temperature, and catalysts—we can harness its potential more effectively.
Whether you’re a scientist or simply curious, the chemistry of HCOOCH3 + H2O offers a glimpse into the intricate world of molecular interactions and their impact on our lives and the universe.